Abstract
Introduction: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) yet the major cause of death remains relapse after transplantation which occurs in 30-70% of patients for whom the prognosis is dismal. Since the 1990's donor lymphocyte infusion (DLI) has been proven able to induce remission after allo-HSCT and the use of therapeutic DLI at relapse has widely increased. The immunological mechanism in DLI is primarily T-cell-mediated graft-versus-leukemia (GVL) effect driven by genetic differences between donor and recipient in minor and major histocompatibility antigens. DLI treatment at relapse can additionally reverse T-cell exhaustion and increase T-cell receptor diversity, both of which are GVL-enhancing mechanisms. Risks and complications with DLI-treatment are primarily graft-versus-host disease (GVHD). Though dose escalation schedules have been suggested to increase the GVL-effect while minimizing the risk of GVHD, uniform therapeutic algorithms are still lacking, treatment is often individually scheduled, and outcome results are often disappointing with reported 2-year overall survival rates at 14-29% in AML relapse patients (Greiner J, Götz M, Bunjes D, Hofmann S, Wais V. Immunological and Clinical Impact of Manipulated and Unmanipulated DLI after Allogeneic Stem Cell Transplantation of AML Patients. J Clin Med. 2019;9(1):39). During the last decade, treatment with the hypomethylating agent azacitidin (Aza) has become another potential treatment in patients with myeloid malignancies. Immunological mechanisms of GVL in Aza-treatment for relapse include epigenetically reactivation of pro-apoptotic pathways and demasking of tumor-antigens while increased expression of regulatory T-cells protects from GVHD. In recent years DLI and Aza have been used for synergistical effect post-HSCT relapse both in patients who are un-fit to receive high-dose cytoreductive therapy as well as consolidation after reinduction. The aim of this analysis is to report results of retrospective single center-study of patients treated with DLI +/- Aza over a period of twenty years.
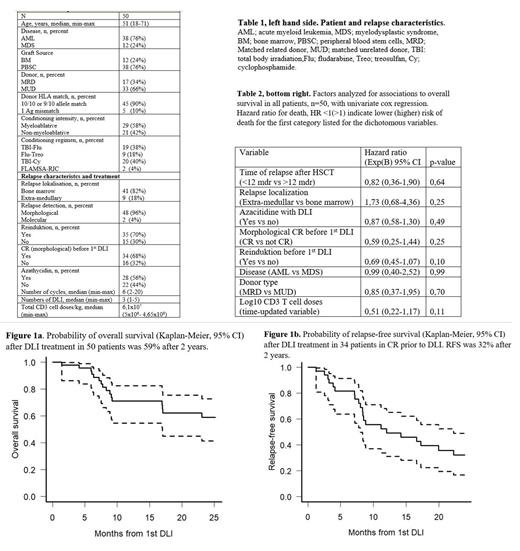
Methods: Between 2001 and 2020 50 adult patients with relapse after allo-HSCT for AML(n=38) or MDS (n=12) were treated with DLI at the Department of Hematology, Transplant Unit, at Rigshospitalet, Copenhagen University Hospital, table 1. Only patients free from active GVHD were selected as DLI-candidates. Median follow-up time was 57 (1-170) months. Reinduction with high-dose chemotherapy was administered in 35 (70%) of patients prior to DLI and 34 (68%) patients were in complete morphological remission (CR) before DLI. DLI-products were unmanipulated and obtained from leukapheresis of unstimulated peripheral blood in matched related or unrelated donors of the original stem cell graft. Patients received a median of 3 (1-5) doses of DLI with median total doses of 6,1x10 7 (5x10 6- 4,65x10 8) CD3 postive T-cells per kg. Aza was used together with DLI from 2012 and administrered in 28 (56%) patients with a median of 6 (2-20) cycles. Reported outcomes are overall survial (OS) and relapse-free survival (RFS) in patients in CR prior to DLI.
Results: At end of follow-up 20 patients were alive, 11 of these in CR and 2 in partial remission. In 7 patients, DLI was discontinued due to the development of GVHD after 1-2 doses, 6/7 of these patients had unrelated donors. Overall, 2 (4%) patients died from GVHD after DLI. Seven patients received a second HSCT after DLI treatment and were censored at this date in survival analyses. Figure 1a+b shows OS in all patients (n=50) and RFS in patients in CR prior to DLI (n=34). 2-year OS was approximately 59% and 5-year OS was 20%. 2-year RFS was approximately 32% and 5-year RFS was 8%. None of the analyzed baseline factors showed significant associations to the probability of OS, table 2, or RFS (data not shown). Reinduktion before first DLI and increasing doses of transplanted CD3 T cell per kg showed trends towards superior survival probability but failed to reach significant levels, possibly due to the limited patient number.
Conclusion: Treatment vith DLI +/- Aza is effective and safe as relapse-treatment after allo-HSCT in myeloid diseases. In selected patients, a short-term (2-year) overall survival of 59% is achieved, and 20% of the patients remain long term survivors.
Fischer-Nielsen: A.F.N. is employee and shareholder of StemMedical A/S, a biotech company working with cell-enriched fat grafting.: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal